NUCYNTA: ONE SOURCE OF RELIEF IN THE ACUTE SETTING1
NUCYNTA: Proven efficacy in post-surgical pain vs placebo.1
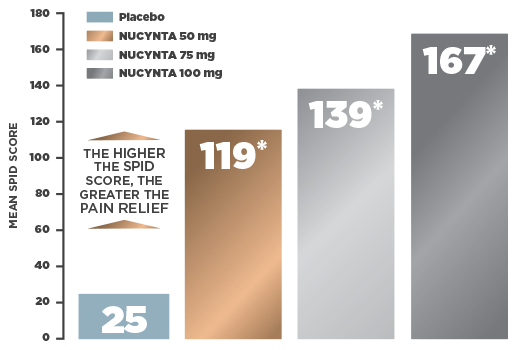
CHANGE IN MEAN PAIN INTENSITY OVER 48 HOURS IN A BUNIONECTOMY STUDY1
 *
P<0.001 based on all comparisons vs placebo.
*
P<0.001 based on all comparisons vs placebo.
- Primary efficacy endpoint: Sum of pain intensity difference (SPID) over the first 48 hours of treatment as measured by the 11-point Numerical Rating Scale (NRS)†
- Primary efficacy analysis: Based on the intent-to-treat (ITT) population who received at least 1 dose of study drug and completed a baseline pain assessment
- Mean baseline pain intensity score: 7.02
Oxycodone IR was included in the study as an active control to confirm the sensitivity of the pain models.1
†An 11-point pain intensity scale, with a score of 0 being "no pain" and a score of 10 being "pain as bad as you can imagine."
Study design:
In a randomized, double‑blind, active‑ and placebo‑controlled, parallel, multicenter, phase 3 study, subjects with moderate to severe pain following bunionectomy (n=602, ITT) were selected to evaluate the efficacy and safety of NUCYNTA. Subjects were randomized to receive NUCYNTA 50 mg, 75 mg, or 100 mg, oxycodone IR 15 mg, or placebo. Subjects received study drug every 4 to 6 hours over 3 days on an inpatient basis. The primary efficacy endpoint was the SPID over the first 48 hours.1
NUCYNTA: Proven efficacy for moderate to severe acute pain vs placebo3,4
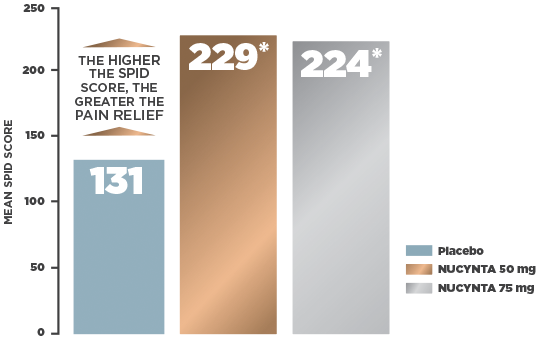
CHANGE IN MEAN PAIN INTENSITY OVER 5 DAYS IN AN END-STAGE DEGENERATIVE JOINT DISEASE STUDY3,4
* P<0.001 based on all comparisons vs placebo.
Study Design +- Primary efficacy endpoint: Sum of pain intensity difference (SPID) over the first 5 days of treatment as measured by the 11-point Numerical Rating Scale (NRS)†
- Primary efficacy analysis: Based on the intent-to-treat (ITT) population who received at least 1 dose of study drug and completed a baseline pain assessment using the last observation carried forward (LOCF) method
- Mean baseline pain intensity score: 6.74
Oxycodone IR was included in the study as an active control to confirm the sensitivity of the pain models.3,4
†An 11-point pain intensity scale, with a score of 0 being "no pain" and a score of 10 being "pain as bad as you can imagine."
Study design:
In a multicenter, randomized, double‑blind, active‑ and placebo‑controlled, parallel group, phase 3 study, subjects who were candidates for joint replacement surgery due to end‑stage joint disease (n=659, ITT) were selected to assess the efficacy and safety of NUCYNTA. Patients were randomized 1:1:1:1 to receive NUCYNTA 50 mg, 75 mg, oxycodone IR 10 mg, or placebo. Patients received study drug every 4 to 6 hours during waking hours for 10 days. The primary efficacy endpoint was the SPID over 5 days.3
- Daniels SE, Upmalis D, Okamoto A, et al. A randomized, double‑blind, phase III study comparing multiple doses of tapentadol IR, oxycodone IR, and placebo for postoperative (bunionectomy) pain. Curr Med Res Opin. 2009;25(3):765‑776.
- Data on file. Collegium Pharmaceutical, Inc. (Bun I).
- Hartrick C, Van Hove I, Stegmann J‑U, et al. Efficacy and tolerability of tapentadol immediate release and oxycodone HCI immediate release in patients awaiting primary joint replacement surgery for end-stage joint disease: a 10‑day, phase III, randomized, double‑blind, active‑ and placebo‑controlled study. Clin Ther. 2009;31(2):260‑271.
- Data on file. Collegium Pharmaceutical, Inc. (EDJD).